November 30, 2020:
Where We Are Today And When Will New Vaccines Be Approved?
by Bob Kordella, R.Ph., MBA, Chief Clinical Officer
Worldwide there were 48 COVID-19 vaccines undergoing various stages of clinical evaluation as of November 12, 2020. Of these, six candidates have received funding from Operation Warp Speed (OWS), the U.S. government’s program to fast track COVID-19 vaccines and therapies, and five of the six candidates already have or are likely to seek emergency use authorization (EUA) from the Food and Drug Administration (FDA) between now and early 2021. An EUA permits a drug maker to bring a product to market at an earlier stage in the FDA’s review process than is typical. It is important to remember that an EUA is extraordinary, and it does not constitute unconditional FDA approval.
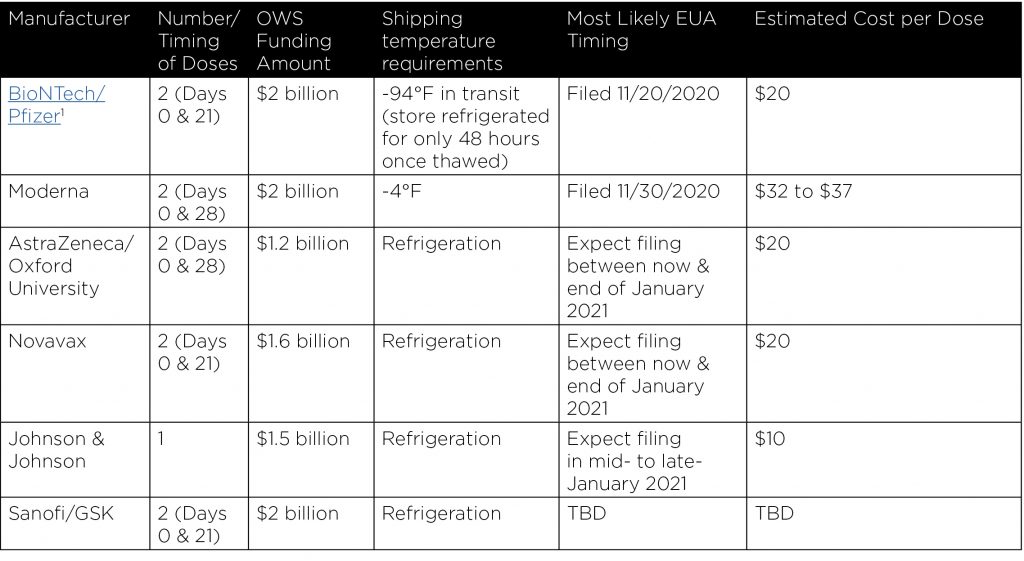
The FDA has set a meeting date of its Vaccines and Related Biological Products Advisory Committee for December 10, 2020, to discuss an Emergency Use Authorization (EUA) of the BioNTech/Pfizer vaccine candidate, and Moderna reports that it will meet with the FDA one week later, on December 17, 2020, to discuss its Emergency Use Authorization application. The meeting link for the Moderna meeting has not yet been posted. Vice President Mike Pence told the nation’s governors during a conference call with the White House Coronavirus Task Force on Monday, November 30th that some doses of the COVID-19 vaccine could begin to be distributed as early as mid-December, presumably referring to the Pfizer vaccine candidate.
The BioNTech/Pfizer and Moderna vaccine candidates, though first, also have the most challenging shipping and cold storage requirements which will limit the locations at which they may be administered. Administration of these two early vaccines will likely require reliance upon existing state & local public health infrastructure as they are unlikely to be amenable to workplace, health plan, or community administration on a wide scale.
Over time, as additional vaccine candidates seek and gain FDA authorization/approval, the number of administration venues will be expanded as later vaccine candidates do not have the challenging cold shipping and storage requirements, particularly of the extreme nature of the BioNTech/Pfizer vaccine candidate. Later vaccines will likely be available in many or most of the same locations that, today, offer annual flu shots. So, while the first vaccines to receive EUA from the FDA will make news, they will not be easy to deploy, particularly for employers and health plans.
Who will be eligible to receive vaccines first and how will vaccines be distributed?
The CDC has published its COVID-19 Vaccination Program Interim Playbook for Jurisdiction Operations to provide guidance to state and local public health officials. CDC has also announced that it will hold a meeting of its Advisory Committee on Immunization Practices (ACIP) on December 1, 2020, to finalize its guidance to state and local health departments as to which populations to include in Phase 1a of COVID-19 vaccine distribution. CDC has previously explained that final prioritization decisions will be made at the time of an EUA for each vaccine. The final prioritization plans will be based on recommendations from the CDC’s Advisory Committee on Immunization Practices (ACIP), as well as recommendations from the National Academy of Medicine advisory panel. The initial playbook remains a helpful guide into what the CDC will likely be implementing as the vaccine approval process is completed. It encourages officials to consider which
portions of the population should receive the vaccine first, and that officials develop a phased approach to vaccine distribution to accommodate an evolving supply of vaccines.
For example, it’s likely that the following groups will be prioritized to receive the first vaccines that are
available:
- Healthcare personnel
- Non-healthcare essential workers
- Adults with high-risk medical conditions who possess risk factors for severe COVID-19 illness
- People 65 years of age and older including those living in long-term care facilities.
Distribution of vaccines will be accomplished in phases as more vaccines become available. These phases will likely occur as follows:
Phase 1: Potentially limited supply of COVID-19 vaccine doses available. Efforts will be concentrated on reaching the initial prioritized populations mentioned above.
As an example of the scale needed in Phase 1, it is estimated that there are currently 19 million high-risk healthcare workers and first responders in the U.S. and this alone group would require 38 million doses. We generally expect this phase to begin before the end of December 2020.
Phase 2: A larger number of vaccine doses become available. Focus will be on ensuring access to vaccines for all remaining prioritized populations, as well as expanding into critical infrastructure populations , and a broader distribution network will be required. We generally expect this phase to begin in late January though early February of 2021.
Phase 3: Sufficient supply of vaccine doses for entire population available. Best estimates are that Phase 3 will arrive, barring any unforeseen distribution or supply chain delays, at some point in the second quarter of 2021.
Will an annual COVID-19 vaccine be necessary?
We don’t know yet and it is one of the reasons that unconditional FDA approval isn’t being granted at this time. There are certain questions, and this is one of them, whose answers cannot be accelerated and can only be determined with the passage of time. We will know more about the durability of vaccine protection as experience with these vaccine candidates evolves through their use in ongoing clinical trials and under EUAs.
In addition, the initial clinical trials results that have been reported were focused primarily in an adult population, so ongoing trials will include children. Some other questions that remain include how well the vaccines will work in specific population groups, as well as understanding more about people who are infected with COVID-19 but are asymptomatic.
What else should we be thinking about in the meantime?
Understand what your existing vaccine coverage process entails – whether it exists under the medical benefit only, under the pharmacy benefit only, or under both. Anticipating a role for widespread pharmacy and outpatient vaccination clinics as vaccine distribution & availability ramps up, your vaccine benefit structure ought to facilitate as broad a level of access as possible, under both the pharmacy and medical benefits. Now is the perfect time to understand your existing vaccine coverage status, and to make any changes necessary to facilitate broad access.
The pace of change is accelerating, and we will keep you posted as things evolve.

Bob Kordella, R.Ph., MBA
Chief Clinical Officer Vice President
Bob has more than 35 years of diverse experience in the pharmacy industry. Over the course of his career, Bob has led clinical and PBM operations teams in successfully managing more than $4 billion in annual drug spend. This was also while limiting per-member-per-year spending growth to levels that have simultaneously drawn industry acclaim and consistently high levels of member and payer satisfaction.
Aducanumab For Alzheimer’s Disease:
Its Past, Present, & Potential Future
by Robert Kordella, RPh, MBA, Chief Clinical Officer
Prior to the COVID-19 pandemic, no product with the possible exception of Roctavian, the gene therapy for use in Hemophilia A, loomed larger in the specialty pipeline than aducanumab, a potential new treatment for Alzheimer’s disease (“AD”), a disease that has persistently defied all prior attempts to eliminate or markedly slow the inevitable cognitive decline for which it is so infamous. Considering the relatively wide prevalence of AD (an estimated 5.8 million Americans of all ages were living with Alzheimer’s dementia in 2019) combined with current uncertainty over the number of those individuals whom the FDA may deem appropriate candidates for aducanumab treatment, along with the drug’s eventual price should it be approved, the potential impact to Pharmacy benefit plans becomes starkly evident.
While aducanumab may hold out promise for treating a disease that has historically been resistant to effective, long-term drug treatment, you may not know that it has had an eventful past year-and-a-half that’s worth understanding as it pursues FDA approval. Let’s take a look at aducanumab’s recent past, its current status, and its potential future role & impact in the treatment of AD.
The Past
One of the most popular theories of the root cause of AD involves the disruptive growth of a protein called beta-amyloid in the brain. Aducanumab was developed to prevent or slow the growth of beta-amyloid, with the hope that AD cognitive decline could be slowed if not eliminated.
Biogen, the manufacturer of aducanumab, was in the process of conducting two separate clinical trials on aducanumab when, in March of 2019, the company announced that it was halting both trials immediately because an independent review board had determined that, based on its review of the available data, aducanumab was no more effective than placebo in treating AD.
Seven short months later, however, Biogen announced in October of 2019 that a new analysis did show a benefit of aducanumab vs. placebo, and that it would seek FDA approval based on this new analysis. This new analysis included more data than was available to the independent review board when it ordered the shutdown of the two clinical trials, but it focused only on patients who had received higher doses of aducanumab, not all patients in both trials – essentially a subset of the whole dataset. Biogen indicated that this new analysis was conducted in consultation with the FDA.
The Present
On November 6, 2020, the FDA convened a meeting of outside experts having expertise in the bioscience & treatment of AD to review Biogen’s data and vote on whether the drug should be approved based on that review. The panel voted overwhelmingly against approval, saying evidence from one positive Biogen study is inadequate to demonstrate the drug’s safety & efficacy in light of conflicting results from a second Biogen trial. In reaching its conclusion, the panel addressed three broad questions:
- Do the data prove aducanumab’s broad or limited effectiveness?
- Is aducanumab safe (since one-third of patients receiving high doses in the clinical trials developed a particular type of swelling of the brain)?
- Does the potential benefit of aducanumab outweigh its potential risks?
In light of the panel’s negative vote, it is important to note that the FDA is not required to follow the panel’s recommendation and may decide to approve aducanumab anyway.
The Future
What does the future hold? Estimates peg the number of mild/early AD patients in the U.S. at 2.2 million. If one-third of them were to receive aducanumab at a price of $50,000 annually (Note: Biogen has not confirmed what pricing it may adopt if aducanumab is eventually approved), sales of aducanumab would amount to ~$37 billion annually in the U.S. For comparison’s sake, Humira’s U.S. sales in 2019 amounted to $20 billion.
The FDA is expected to decide on aducanumab’s approval status by March 7, 2021. If approved, the following questions remain to be addressed prior to the first prescription for aducanumab being written and filled:
- What limits may the FDA impose on aducanumab’s use in terms of AD severity. Will it be approved for all AD patients, or only for some patients having specific disease severity?
- If FDA approval is limited, will AD caregivers pressure MDs to prescribe for off-label use to benefit their loved ones? Ought such off-label use be paid for by pharmacy benefit plans?
- What price will Biogen set for aducanumab?
- What supporting clinical documentation may FDA establish to justify treatment with aducanumab (PET scans, spinal taps, etc.), and how much will those diagnostic requirements indirectly add to the total cost of care with aducanumab?
- How will FDA ensure that doses of aducanumab are high enough to be effective, but low enough to avoid the brain swelling side effect observed in clinical trials?
These are some of the many questions that aducanumab brings with it, and we will continue to monitor its progress through the FDA approval process.

Robert Kordella, RPh, MBA
Chief Clinical Officer
Bob has more than 35 years of diverse experience in the pharmacy industry. Over the course of his career, Bob has led clinical and PBM operations teams in successfully managing more than $4 billion in annual drug spend. This was also while limiting per-member-per-year spending growth to levels that have simultaneously drawn industry acclaim and consistently high levels of member and payer satisfaction.
Specialty Medications To Treat Chronic Inflammatory Skin Conditions Among Top 10 In Spend For Most Plan Sponsors
by Caroline Atwood, Pharm D., Vice President | Team Lead
Atopic dermatitis (eczema) is a chronic inflammatory condition that makes your skin red and itchy. Other symptoms of atopic dermatitis may include scaly dry skin, rash with clear fluid, painful cracked skin that bleeds, and darkening of skin around the eyes. Atopic dermatitis may be accompanied by hay fever or asthma. It is the most common type of eczema affecting ~15-20% of children and up to 10% of adults worldwide. Atopic dermatitis flares periodically and may be triggered by certain allergens or stress.
You can’t cure atopic dermatitis, but you can treat the symptoms. Medications commonly used to treat atopic dermatitis include steroid creams or ointments for mild flares or oral steroids for more severe symptoms. These medications are relatively inexpensive. Dupixent, an injectable biologic, slows down the immune system and eases the inflammation but can cost over $30,000 per year. The utilization has been increasing across many plan sponsors. They are seeing the cost to treat this condition skyrocket.
The atopic dermatitis specialty pipeline includes eight new drugs. Four Janus kinase (JAK) inhibitors with anticipated approvals in 2021; abrocitinib (oral), baricitinib (Olumiant) (oral), ruxolitinib (Jakafi) (topical) and upadacitinib (Rinvoq) (oral). In addition, tralokinumab (SC) an Interleukin-13 inhibitor with anticipated approval in 2021. The other three specialty drugs have 2023 anticipated approval dates.
There are a few things patients can do at home to help with flares. These include warm oatmeal baths, apply skin moisturizers, use a cool mist humidifier, use only mild soap and wear loose fitting clothes. Plan sponsors should ensure they’ve implemented appropriate utilization management to require the use of the topical products and non-pharmacologic products as first line therapy.
Psoriasis
Psoriasis is chronic inflammatory condition which primarily affects the skin and joints. It is estimated to affect 2-3% of the global population. Psoriasis most commonly causes red, itchy scaly patches on the scalp, lower back, and parts of the elbows and knees. Psoriasis tends to go through cycles, flaring for a few weeks or months, then ceasing for a time or going into remission. The exact cause of psoriasis is unknown, and it is thought to have a genetic association. There are several types of psoriasis including Plaque psoriasis affecting about 80% to 90% of psoriasis patients.
Environmental factors can trigger or exacerbate outbreaks of psoriasis in genetically predisposed individuals including weight gain, smoking, alcohol consumption, stress, withdrawal of corticosteroids, HIV infection, and the use of certain medications.
The treatment goal of psoriasis is to find the most effective way to slow cell turnover with the fewest side effects. Treatment selection is personalized to the patient taking into consideration the severity of the disease, efficacy, side effects, comorbidities, and impact on quality of life. The traditional approach is to start with topical creams and phototherapy in patients with skin lesions. Patients with severe psoriasis, or associated with arthritis, may need systemic or biologic therapies from the beginning.
Corticosteroids are the most frequently prescribed medications for treating mild to moderate psoriasis. Light therapy is a first-line treatment for moderate to severe psoriasis and can be used alone or in combination with medications. Oral and injected medications are prescribed for moderate to severe psoriasis or if other treatments haven’t worked. These include steroids, retinoids, methotrexate, cyclosporine and biologics. Biologics alter the immune system in a way that disrupts the disease cycle and improves symptoms of the disease within weeks. Examples include Enbrel, Humira, Stelara, Cosentyx, Otezla and Talz. These biologics are expensive. They must be used in caution because they carry the risk of suppressing your immune system that can increase your risk of serious infections. There are three new drugs for Plaque psoriasis in the pipeline for 2021, two injectable (bimekizumab, mirikizumab) and one oral (deucravacitinib). Upadacitinib (Rinvoq) is an oral drug with an expanded indication for Psoriatic arthritis, anticipated approval April 2021.
There is no question that the cost of treatment for Atopic Dermatitis and psoriasis is a concern for payers. New biologics are highly advertised and may influence patients. Three of the top ten advertised drugs are for psoriasis or atopic dermatitis. Frequent light therapy treatments add up, medications can run into thousands of dollars, and the biologics can run up to $100,000 per year. There are ways to help manage the cost of chronic inflammatory skin conditions for both plan sponsors and patients. Prior authorization, step therapy edits, preferred products, copay maximization programs, site of care optimization, clinical outcomes monitoring, and specialty exclusive fulfillment are managed-care tools to help with clinical appropriateness and cost management. Increased competition with a robust pipeline may help with future costs. In addition, biosimilars coming to market can provide a cost advantage. Enbrel has two approved biosimilars pending launch, anticipated launch 2029. Annual U.S. sales $8B. Humira has six approved biosimilars pending launch, anticipated launch 2023. Annual U.S. sales $18.3B.
Lockton pharmacy consultants are an excellent resource for education on the impact of new products coming to market, biosimilars, and innovative strategies to help manage client and patient costs.

Caroline Atwood, Pharm D.
Vice President | Team Lead
Caroline is a Vice President, Team Lead at Lockton Dunning Series where she works with employers and health plans to develop solutions to effectively manage their pharmacy benefits and deliver the highest quality of healthcare. She has a proven track record for building and maintaining solid executive level relationships with key decision makers in healthcare and payer communities.
Before joining Lockton, Caroline was a Vice President, Consultant Relations OptumRx for UnitedHealthcare/UMR for 11 years.
PBM Contracts – The Devil Is In The Details
Drug prices have been one of the primary topics politically this election season and a regular part of the news cycle for several years now bringing consumer awareness to an all-time high. For decades pharmacy spend flew under the radar for many plans and employers but that is no longer the case. In fact, as a percentage of overall healthcare spend, pharmacy is now approaching the 25% range and employers are feeling the pressure when it comes to designing a benefits package that offers their employees value while remaining affordable and sustainable for the company. In today’s world, one employee or dependent can change the entire trajectory of an employer’s healthcare spend in large part due to incredibly expensive drug therapies on either the medical or pharmacy plan.
Carrier and PBM pharmacy contracts include complex financial arrangements with carefully constructed terms and conditions designed to make their offer appear better than really is. Definitions, rebates, and pricing arrangements leave plenty of room for ambiguity and opaqueness. Generally, there are two standard pricing arrangements in the pharmacy benefit space – Spread (traditional) and Pass Through. Spread pricing means that the PBM’s profits are determined by the difference between what the PBM bills the plan and what the PBM pays the pharmacy. Pass through pricing means the PBM bills the plan sponsor what they pay the retail pharmacy.
Far from an all-inclusive list, these are some of the things to consider when negotiating Pharmacy Benefit contracts with Carriers and PBMs:
- Most contracts are based on an aggregate percentage discount from Average Wholesale Price (AWP). Pharmaceutical manufacturers increase the AWP once to twice per year. As the AWP of a drug increases, the PBM’s portion of spread per claim increases as well. Because of this it’s important to ensure your contract has increasing discounts each year as well as market check provisions during a multi-year contract term.
Example
- 1/1/20 Drug AWP = $100
- Plan Discount AWP -18%, Pharmacy Discount AWP -20%
- Plan pays $82, Pharmacy is paid $80, PBM retains $2
- 7/1/20 Drug AWP increases to $120
- Plan discount AWP -18%, Pharmacy Discount AWP -20%
- Plan pays $98.40, Pharmacy is paid $96, PBM retains $2.40
- Generic drugs are less costly for plan sponsors but remain very profitable for PBMs. This creates the potential for aligned incentives but must be watched closely. Generic pricing is also generally based on an aggregate discount off AWP however PBMs manage generic pricing through the use of MAC (Maximum Allowable Cost) lists and it is relatively rare that a generic drug claim will pay based on an AWP discount.
This list of drugs is maintained by each individual PBM and they have full discretion when it comes to the management of this list. The original intent of a MAC list was to standardize the reimbursement of generic drugs which are available from several manufacturers so that pharmacies would not be incentivized to bill for certain more expensive versions of the generic. This list sets a price by drug, not by manufacturer and is meant to set pricing based on average actual acquisition costs of these less expensive drugs rather than based on an AWP that everyone knows is falsely high (typical AWP discounts for generic drugs are in the 80-84% range). - High cost generic drugs have created a challenge for PBMs as they have made it more difficult for PBMs to achieve their contracted discount guarantees. This has led to PBMs pushing for language in their contracts that allows them to consider high cost generic drugs to be counted as brand name drugs from a discount guarantee perspective. This practice greatly benefits the PBM and impacts employers in a negative way while allowing PBMs to bid with rates that appear to be competitive when just focusing on the numbers and not the contract terms. Plan sponsors need to closely look at the definition of what is included in the generic guarantee. From a plan design perspective, some PBMs offer strategies to drive to utilization of lower net cost generics by creating a high cost generic tier on the formulary and by doing outreach to educate members and physicians on opportunities for cost savings in this area.
- Rebate payments to plan sponsors are typically based on a minimum guarantee per Brand name prescription claim. They are not tied to the price of the drug and are not even directly tied to the pharmaceutical manufacturer rebate contracts associated with specific drugs. PBMs retain all rebate revenue that they are able to generate that is above and beyond the minimum guarantees they offer in the contract unless there is language in the contract that obligates the PBM to pass along the full value of the payment. There are often hidden payments from the manufacturer that aren’t categorized as a “rebate” within the terms of the contract. Rebates remain a black box of uncertainty in the industry leading to distrust and frustration for employers.
- Formulary is the single biggest driver of member utilization patterns and the basis for rebate payments. Since PBMs benefit greatly from rebate revenue, there can be misaligned incentives that must be considered and managed appropriately. Drugs that offer very little, if any, clinical advantage are added to formularies on a regular basis simply to increase rebate yield for the PBM. This practice has led to higher rebate guarantees but also higher net cost for employers. It is imperative that formulary is considered when evaluating bids in the market to encourage less rebate chasing and more programs that drive to lowest net cost.
- Specialty drugs continue to be at top of mind for plan sponsors. From a contracting perspective, PBMs have begun to exclude Limited Distribution and Biosimilar Specialty Drugs from discount and rebate guarantees within the small print of their contracts. These exclusions can account for 40% of specialty spend and must be accounted for when comparing bids to one another to ensure no PBM is unfairly advantaged. PBMs have also created programs to capture manufacturer coupon value on specialty drugs for plan sponsors and members. These programs require exclusive specialty arrangements and shared savings scenarios. While there are significant savings for clients, it’s important to understand the revenue opportunity for the PBMs as well.
Aligned incentives should be a high priority when considering Carrier/PBM partners. Having a consultant that is vendor agnostic is imperative as they help employers navigate the incredibly complex system of Pharmacy Benefits. Becoming more educated on the revenue streams helps employers understand why PBMs make the decisions they make, whether it is a fully insured, or self-funded carved in or carved out plan. Patient care and clinical outcomes should be the focus of health care decisions, not rebates and undisclosed profit margins. Lockton pharmacy experts can help you navigate the complex world of pharmacy contracting and management.

Jenn Dimura, R. Ph.
Vice President, Senior Pharmacy Consultant
Jenn Dimura has over 20 years of versatile pharmacy and PBM account management experience with both managed care and retail experience. Jenn has worked in account management providing client-specific business plans focusing on client goals, performance expectations, growth in membership and profitability. Her knowledge of financial and pricing models to analyze and enhance client clinical scenarios provide illustration of various benefit strategies addressing a multitude of organizational goals.
