Humira Savings on the Horizon?
by Robert Kordella, RPh, MBA, Chief Clinical Officer
In 2021, Humira (adalimumab) amassed $21 billion in sales in the U.S. for its manufacturer, AbbVie, making it the year’s most costly product, excluding Pfizer’s COVID-19 vaccine, Comirnaty® ($37 billion). Humira became the world’s top-selling drug in 2012 and has remained No. 1 ever since. In 2023 a phased rollout of biosimilar versions of Humira will occur, which could result in savings to plan sponsors on this high-cost medication, but there are a lot of moving parts that will impact the timing and amount of savings potential.
As the below chart attempts to succinctly illustrate, there are currently ten manufacturers that have either already received or are working to receive FDA approval and AbbVie legal agreement to launch biosimilar alternatives to Humira. Several manufacturers are pursuing dual concentrations (the original 50 mg/mL concentration and the newer 100 mg/mL concentration) as well as interchangeability status at launch. Only one product (Amgen®’s 50 mg/mL, non-interchangeable product, Amjevita™, will launch on Jan. 30, 2023, with 180 days of exclusivity, so despite the volume of intensive press coverage that it will receive, it is not expected to attain much uptake or deliver material savings. Savings opportunities will be associated with the flood of product launches that will begin on or around July 1, 2023.
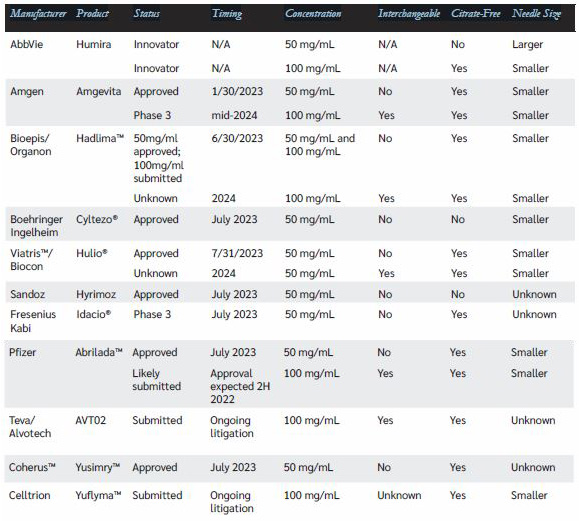
As you might expect, AbbVie is not expected to give up a $21 billion market without a fight to “defend the fort.” While AbbVie is understandably keeping its plans confidential, the tools available to them to preserve as much market share of Humira in the face of biosimilar competitors will include:
- Lowering the list price
- Increasing rebates
- Increasing purchase discounts to large buyers
- Maintaining or enhancing patient assistance programs
- Requiring bundled (“all or none”) full-line AbbVie rebate contracts
- Maintaining aggressive 340B program pricing
- Switching patients to other products in their portfolio — Skyrizi® and Rinvoq®
Bottom line:
- The materiality of making the right decision with regard to biosimilar Humira is the single largest decision that has ever faced pharmacy benefits plans. We will continue to assist you in navigating this process over the next year and we continue to encourage you to ask your PBM or carrier for updates on their plans and progress for biosimilar Humira at every opportunity.
- Owing to the complexity of the decision points, early adopters are not likely to be rewarded with the best deal, so expect final plans to be delayed well into the first half of 2023, following the first biosimilar launch in January 2023.
- No pharmacy benefits plan ought to be paying a higher net cost for adalimumab in July 2024 than they were in July 2023.
- Parting thought: Biosimilar versions of Humira launched in other parts of the world produced a 23% decline in Humira sales in those locations, so the potential for biosimilar versions of Humira to produce similar savings in the U.S. is certainly on the horizon.
Enjoy the savings, and please reach out to your Excelsior Solutions account team if you require any assistance understanding the timing and quantifying the impact of these upcoming generic launches to your pharmacy benefit plan. We are always happy to help.

Robert Kordella, RPh, MBA
Chief Clinical Officer
Bob has more than 35 years of diverse experience in the pharmacy industry. Over the course of his career, Bob has led clinical and PBM operations teams in successfully managing more than $4 billion in annual drug spend. This was also while limiting per-member-per-year spending growth to levels that have simultaneously drawn industry acclaim and consistently high levels of member and payer satisfaction.
Are Limited Distribution Drugs Limiting Your Savings?
by Jenn Dimura, RPh, Vice President and Director of Pharmacy Solutions
The ever-evolving pharmacy industry continues to present challenges to plan sponsors to stay on top of concepts and minutiae they never imagined would have to be part of their daily thought process. The acronyms, the complicated contracts, misaligned incentives, and complex clinical therapies make it a difficult world to navigate without a trusted advisor who only has your best interest in mind. Even with years of experience in this field of work, it is a constant barrage of new information and new tactics being used to increase the face value of a PBM contract.
One relatively new phenomenon in the industry is the propensity for manufacturers to deem their newly approved therapies as “limited distribution” drugs. A limited distribution drug (LDD) is a therapy that is only available through a small number of pharmacies around the country. These drugs cannot be obtained locally or often through your preferred specialty or mail order pharmacies. They are typically high-cost specialty drugs which often do not offer rebates due to the lack of competition they experience. Most new drug approvals today are specialty drugs for the treatment of rare diseases. This means we will continue to see an increase in the number of LDDs on the market. The question is, how does that impact health plans and employers.
When a plan sponsor hears the term limited distribution drug, it might be difficult to understand why this term matters or results in a material impact to them. Across the Excelsior Solutions’ book of business, 16% of claims and 31% of spend on specialty is for LDDs. The impact varies depending on the specific utilization of a plan sponsor’s population but should be considered when reviewing a pharmacy contract. Often PBMs will “exclude” LDDs from both discount guarantees as well as rebate guarantees. This exclusion is very impactful when analyzing the value of an overall deal, and if it is not considered, the rebates and specialty discounts will appear to be far more aggressive than they really are.
As an example, if the aggregate specialty drug discount guarantee is average wholesale price (AWP) minus 18% and LDDs are processing at AWP minus 14%, the PBM would benefit by being able to exclude those drugs that process at a lower discount from the guarantee calculation. It works the same way for rebates.
As the number of LDDs continues to grow, this is becoming a more significant issue as time goes on. Excelsior Solutions’ Pharmacy team evaluates the financial impact of including and excluding LDDs to appropriately analyze each deal. This ensures all inclusions and exclusions are properly reviewed and negotiated so our client’s contracts remain strong and competitive.

Jenn Dimura, RPh
Vice President and Director of Pharmacy Solutions
Jenn Dimura has over 20 years of versatile pharmacy and PBM account management experience with both managed care and retail experience. Jenn has worked in account management providing client-specific business plans focusing on client goals, performance expectations, growth in membership, and profitability. Her knowledge of financial and pricing models to analyze and enhance client clinical scenarios provide illustrations of various benefits strategies addressing a multitude of organizational goals.
The Affordable Insulin Now Act
by Sarah Martin, ASA, MAAA, FCA, CEBS, Senior Vice President
In March 2022 the U.S. House of Representatives voted to approve the Affordable Insulin Now Act, legislation that would cap the patient out-of-pocket cost on insulin for private insurance and Medicare. While the legislation has not made it through the Senate, it would cap member insulin costs at the lower of $35 per month, or 25% of the negotiated price.
Insulin prices have long been a topic of conversation and concern, and many states already have laws around capping the cost of insulin under insurance plans regulated by the state. Over 15 states already have some form of insulin cost cap in place, but not all requirements apply to self-funded employers. ERISA preemption is often cited as protection for self-funded employers from some state requirements, but ERISA preemption would not apply to legislation at the federal level.
Proponents of the Affordable Insulin Now Act argue that many Americans cannot afford their insulin or other prescription medications, and so they go without it. Critics of the bill argue that capping the cost of insulin for members or patients does nothing to solve the problem of overall rising prescription drug prices, or insulin prices.
What could this act mean for employer-sponsored plans?
- In some cases, nothing. Insulin will typically fall under a plan’s preferred brand tier. If the plan design already has a member copay of $35 or less on the preferred brand tier, the plan already complies with this requirement and no changes would be needed.
- In some cases, a change to the plan design. Most employers in our book of business have a preferred brand copay above $35 per month. This act could require plans to lower the copay for insulin in order to comply. Potential challenges associated with lowering the copay for insulin include:
- Added communication and potential confusion for members if a plan must delineate between the copay for insulin, and perhaps a different (higher) copay for other diabetes medications and other preferred brand drugs.
- Some plans may decide to lower their preferred brand copay for all drugs to simplify administration and understanding for members.
- Unique challenges for high deductible health plans, or plan designs with a pharmacy deductible.
- In the deductible or coinsurance phase, applying a cap to insulin complicates administration and member communication. Capping the cost of a drug in the deductible phase contradicts the normal rules associated with a qualified high deductible health plan, so more guidance here will be necessary.
- Some preventive drug lists associated with qualified high deductible health plans contain insulin on the list. In these cases, insulin would either bypass the deductible and go straight to the coinsurance or copay, or insulin may already be free for the member.
Many insulin products offer copay coupons, and members are already using these today at the pharmacy counter. For example, Lantus® offers a copay card that is used in conjunction with commercial insurance.
Members may already be paying less than $35 per month by utilizing this copay card. This act would only serve to lower the amount of pharmaceutical company copay assistance utilized, costing the plan more money, and has no positive impact on the member.
Enhancing the member’s benefit and shifting cost back onto the plan comes at a price. For clients who would have to decrease the plan’s copay to comply, the average resulting cost increase across our book of business is approximately 0.15% to 0.24%.
Depending on your plan design, formulary, and utilization, the cost impact will vary. Your Excelsior Solutions pharmacy consultant can project cost projections specific to your plan. Excelsior Solutions will continue to monitor this bill, as well as other legislation aimed at prescription drug pricing.

Sarah Martin, ASA, MAAA, FCA, CEBS
Senior Vice President
Sarah is a pharmacy actuary with 17 years of experience in employee benefits. She is responsible for pharmacy benefits financial client deliverables and strategy. Her primary areas of expertise include PBM procurement and contracting, cost forecasting, data analysis, specialty drug predictive modeling and emerging pharmacy benefits strategies. Sarah has been a national guest speaker on pharmacy benefits for the Conference of Consulting Actuaries, the Pharmacy Benefit Management Institute and the International Society of Certified Employee Benefit Specialists. She is an Associate of the Society of Actuaries, a Member of the American Academy of Actuaries, a Fellow of the Conference of Consulting Actuaries and a Certified Employee Benefit Specialist. Sarah earned her bachelor’s degree in applied mathematics from the Missouri University of Science and Technology.
